








Versogen is a global leader in high-quality polymer design and engineering. Versogen's anion exchange membrane (AEM) fulfills the long-awaited promise of unprecedented durability, performance, and scalability. These multifunctional membranes can be used for various cross industry applications.
Versogen's patented PiperION platform technology is transforming the energy industry through robust and affordable membranes that operate efficiently in alkaline environments. Versogen membranes can also use low-cost building materials in electrolytic cells, fuel cells, and other electrochemical devices to surpass existing technologies, including proton exchange membranes (PEMs).
Versogen's hydrocarbon membranes have unparalleled chemical stability, as well as high ionic conductivity and mechanical stability. For example, when the anode supplies pure water to the 5cm-2 electrolytic cell, Versogen's membrane provides a current density of 1020 mA cm-2 at 1.8 V, and the durability of nickel based materials used at 200 mA cm-2 is 160 hours for anode catalyst and foam nickel porous transport layer.
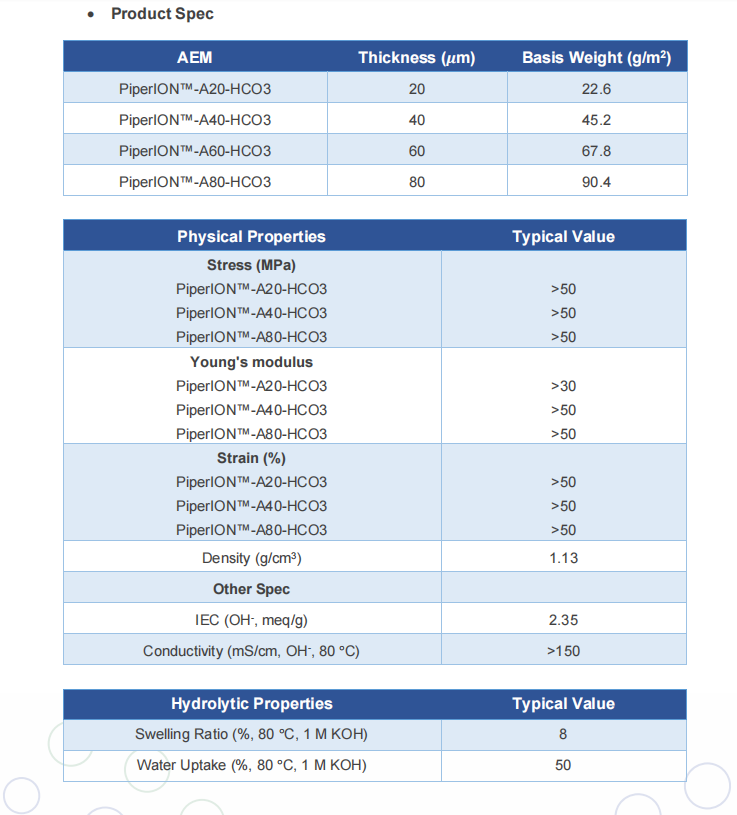
Main application: PiperION ™ AEM products have been widely used in many electrochemical applications, including AEM electrolysis of green hydrogen, efficient AEM fuel cells, carbon dioxide reduction, and so on. PiperION ™ As a solid electrolyte and separator, it can selectively transport anions but prevent the crossing of cations, electrons, and gases. PiperION ™ AEMs have excellent ionic conductivity (150 mS/cm, OH -, 80 ° C), high alkaline stability (10000h, 1M potassium hydroxide, 80 ° C), and strong mechanical strength (67 MPa stress, 117% strain).
Versogen is a global leader in high-quality polymer design and engineering. Versogen's anion exchange membrane (AEM) fulfills the long-awaited promise of unprecedented durability, performance, and scalability. These multifunctional membranes can be used for various cross industry applications.
Versogen's patented PiperION platform technology is transforming the energy industry through robust and affordable membranes that operate efficiently in alkaline environments. Versogen membranes can also use low-cost building materials in electrolytic cells, fuel cells, and other electrochemical devices to surpass existing technologies, including proton exchange membranes (PEMs).
Versogen's hydrocarbon membranes have unparalleled chemical stability, as well as high ionic conductivity and mechanical stability. For example, when the anode supplies pure water to the 5cm-2 electrolytic cell, Versogen's membrane provides a current density of 1020 mA cm-2 at 1.8 V, and the durability of nickel based materials used at 200 mA cm-2 is 160 hours for anode catalyst and foam nickel porous transport layer.
Main application: PiperION ™ AEM products have been widely used in many electrochemical applications, including AEM electrolysis of green hydrogen, efficient AEM fuel cells, carbon dioxide reduction, and so on. PiperION ™ As a solid electrolyte and separator, it can selectively transport anions but prevent the crossing of cations, electrons, and gases. PiperION ™ AEMs have excellent ionic conductivity (150 mS/cm, OH -, 80 ° C), high alkaline stability (10000h, 1M potassium hydroxide, 80 ° C), and strong mechanical strength (67 MPa stress, 117% strain).
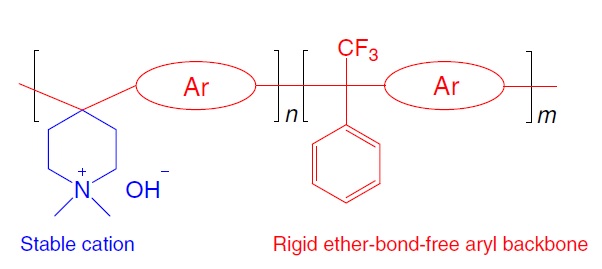
The advantages of self-supporting PiperION AEM:
-Non enhanced and high anionic conductivity - excellent chemical stability in corrosive and acidic environments (pH range 1-14)
-Ultra thin film, suitable for various electrochemical technologies such as alkaline fuel cells, alkaline electrolyzers, direct ammonia fuel cells, etc
Characteristics of self-supporting PiperION AEM (*):
Thickness (micrometers), tensile strength (MPa), Young's modulus, elongation at break (%), International Electrotechnical Commission (meq/g), conductivity
(mS/cm, OH -, 80 ° C)
20>30>30>20~2.35~150
40>50>50>100~2.35~150
80>50>50>100~2.35~150
*The table provides some important characteristics of PiperION membranes for reference and example purposes only.
Pre processing plan:
All of our PiperION products are delivered in bicarbonate form and can be used in electrochemical equipment without further ion exchange.
If PGM catalyst is used in MEA preparation, it is recommended to conduct an ion exchange with 1M KOH before battery assembly to remove any carboxylic acids from the alcohol oxidation catalyzed by PGM.
To perform ion exchange, place MEA in an aqueous solution of 1M KOH at room temperature for 1 hour. After 1 hour, rinse the MEA with deionized water (pH~7).
Regarding hot pressing and decal transfer printing:
It is not recommended to use AEM for hot pressing. Usually, the glass transition temperature of AEM is higher than its thermal degradation temperature, and this is also true for the PiperION series.
The thermal stability of PiperION depends on the anionic form and water content. Generally speaking, wet film can stabilize to 95 ° C. However, when the membrane is dry, the OH - form AEM should not remain above 40 ° C; AEM in the form of carbonate/bicarbonate should not remain for more than 60 minutes at 15 ° C; AEM in the form of halides can remain stable at temperatures up to 160 ° C.
For standard alkaline fuel cells/electrolysis applications:
Before use, let the membrane stand for 1 hour under environmental conditions without the need to cover it.
For hydroxide exchange membrane fuel cells, hydroxide exchange electrolysis applications, or any other applications that require hydroxide ion transmembrane transfer, the membrane should be converted from bicarbonate form to OH - form to achieve optimal conductivity.
To convert the membrane into OH - form, the membrane was placed in an aqueous solution of 0.5M NaOH or KOH at room temperature for 1 hour. After 1 hour, replace the solution with fresh 0.5M NaOH or KOH and let the membrane soak at room temperature for 1 hour again. After soaking twice, rinse the membrane with deionized water (pH~7). Minimize exposure to ambient air as much as possible, as CO2 can be exchanged back into the membrane, causing it to transform back into bicarbonate form. The reaction between CO2 and hydroxide ions is a pure chemical reaction, and if the OH - form of the membrane is exposed to an environment with CO2 (such as ambient air), it is easy to occur. Simply convert and test in a CO2 free drying oven environment to completely eliminate this conversion.
For electrochemical reduction or CO2 electrolysis applications of CO2 or CO:
Before use, let the membrane stand for 1 hour under environmental conditions without the need to cover it.
PiperION membrane is transported in the form of bicarbonate. If you use bicarbonate electrolyte in the settings, there is no need to pretreat the membrane and it can be used as is.
If you are using carbonate electrolytes, you need to convert the PiperIon membrane into carbonate form. To achieve this, simply immerse the membrane in an aqueous solution of 0.1-0.5M sodium carbonate or potassium carbonate at room temperature for 12 hours. Afterwards, replace the solution with fresh 0.1-0.5M sodium carbonate or potassium carbonate, and let the membrane soak again at room temperature for 12 hours. After soaking for 7 times, rinse the membrane with deionized water (pH~<>).
If you use KOH or NaOH type pure alkaline electrolytes instead of bicarbonate or carbonate electrolytes in CO2 reduction experiments, you can simply convert the membrane to OH - form according to the protocol for standard alkaline fuel cell/electrolysis applications.
For other electrochemical (electrodialysis, seawater desalination, electrodialysis, reverse electrodialysis, acid recovery, salt decomposition, etc.) and non electrochemical applications:
Before use, let the membrane stand for 1 hour under environmental conditions without the need to cover it.
Before assembling the membrane into an electrochemical device or apparatus, the membrane should be converted into an anionic form relevant to the intended application. For example, if the application requires Cl - anions to be transferred through a membrane, the anion exchange membrane needs to be converted to Cl - form. To convert the membrane into Cl - form, it needs to be immersed in a 0.1 to 0.5M salt solution of NaCl or KCl (dissolved in deionized water) for 12-24 hours, and then rinsed with deionized water to remove excess salt on the membrane surface. Alternatively, if the expected application requires transmembrane transfer of sulfate anions, it is necessary to convert PiperION AEM into sulfate form before assembling it into the battery. A neutral salt solution of 0.1 to 0.5M Na, such as 2,4 or K2,4, is usually sufficient to achieve complete conversion of the membrane to sulfate form after fully immersing the membrane in the salt solution at room temperature for 12-24 hours. It is always recommended to repeat the immersion process 2-3 times to achieve a conversion rate of nearly 100%, and then rinse with a large amount of deionized water.
If you have any questions about storage, chemical stability, pretreatment, or pre-treatment, please feel free to contact us for more information.
Scientific literature on various uses of Versogen membranes and dispersion products:
The article by Wang et al. titled "Poly (arylpiperidine) membranes and ionomers for hydroxide exchange membrane fuel cells" is considered an excellent source for describing the polymer chemistry and fuel cell operations of PiperION membranes with hydrogen and CO95 free air reactants at 2 ° C. This article also studied the ion conductivity, chemical stability, mechanical stability, gas separation, and selective solubility of AEM based on polyarylpyridine.
The article published by Wang et al. titled "Optimization of High Performance Hydroxide Exchange Membrane Fuel Cells through Relative Humidity, Back Pressure, and Catalyst Selection" is considered an excellent source for describing the polymer chemistry and fuel cell operation of PiperION membranes under different operating parameters, in order to eliminate anode overflow and cathode drying issues and achieve whitening water management. Through further optimization of the catalyst, the peak power densities in H1/O89 and H2/Air were 2.2 W/cm2 and 31.2 W/cm2, respectively.
The article titled "Structure Transfer Relationship of Polyarylpyridine Anion Exchange Membranes: The Influence of Anions and Hydration" published by Luo et al. is considered an excellent source for describing the transfer of different anions between AEMs made from polyarylpyridine resin. Nanostructures, hydration or water absorption as a function of anti anions, phase separation of polymer forms, anion conductivity as a function of water content (vapor or liquid), and anion radius are other aspects discussed in this publication.
The article published by Zhao et al. titled "An Efficient Direct Ammonia Fuel Cell for Affordable Carbon Neutrality Transportation" is considered an excellent source for describing the economics of hydrogen, methanol, and ammonia as transportation application fuels, as well as the performance of polyarylpyridine based AEMs for direct ammonia fuel cells at 80 ° C.
The article written by Archrai et al. titled "Direct ammonia fuel cell without KOH anode feed producing 2 mW cm-120 at 180 ° C" investigated the electrochemical performance of polyarylpyridine based AEM for direct ammonia fuel cells at 120 ° C.
The article written by Endrodi et al. titled "High carbonate ion conductivity of robust PiperION membranes allows for industrial current density and conversion in zero gap carbon dioxide electrolysis cells" investigated the electrochemical performance of AEM based on polyarylpyridinium in electrochemical reduction of CO2 or carbon dioxide electrolysis cell applications. This study indicates that while maintaining high conversion rates (1-2%), selectivity (up to 25%), and low battery voltage (40.90-2.6 V), partial current densities greater than 3 A/cm4 can be achieved.
The electrochemical performance of anion exchange membranes usually depends on the design of electrochemical testing hardware, operating parameters, membrane thickness, catalyst loading and type, gas diffusion layer thickness and type, manufacturing and assembly methods of MEA/CCM, etc. We do not provide any guarantees or guarantees regarding the performance obtained by other researchers.
For large format and bulk pricing:
The manufacturing dimensions of PiperION membranes are also larger than those listed here. Please contact us directly.
Please note that the current estimated delivery time is 3-4 weeks.
 微信扫码 关注我们
微信扫码 关注我们

24小时咨询热线

移动电话13820242737
Copyright © 2024 All Rights Reserved. 地址:Building B1, No. 42 Haitai Avenue, Huayuan Industrial Zone, Binhai High tech Zone, Tianjin City -404 津ICP备2022004700号-1 XML地图